The FDA Quality Report for FY2020: An analysis
FDA recently published its quality report for FY 2020 which presents us a very interesting collection of observations, trends, and programs. Read more about it here.
Interesting Pharma Compliance Insights
Covering the fiscal year 2020 (Oct 2019 to Sep 2020), about half a year under pandemic conditions, giving us insights into Covid’s influence on regulatory observations and actions[1].
https://www.fda.gov/media/151561/download
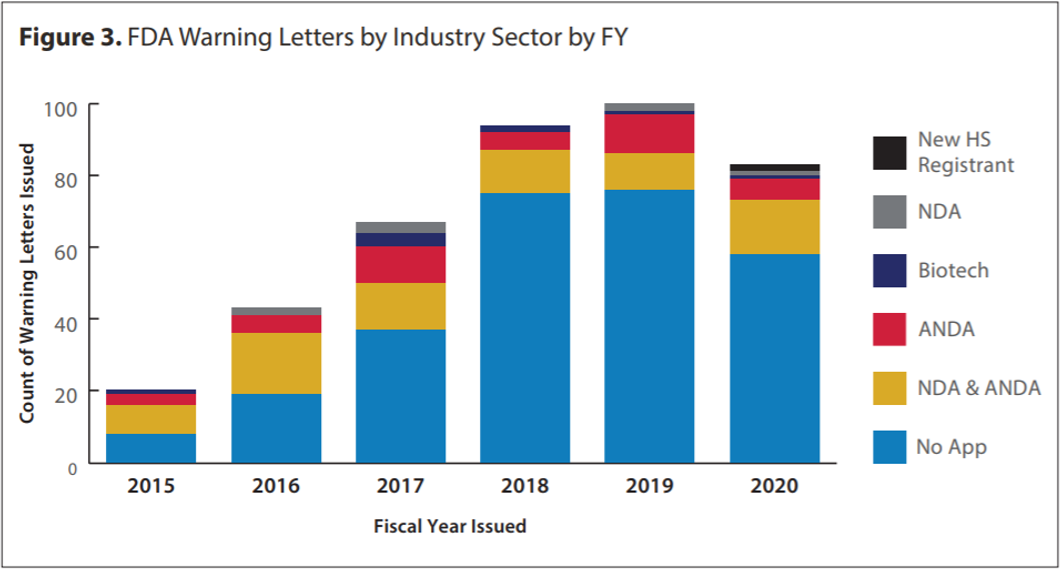
Fewer Warning Letters were issued, and OTC products remained dominant
One trend we observed in the past five years is the increasing proportion of non-application over-the-counter products (OTCs) [2]. Presumably, this trend was further strengthened during the pandemic since many new players entered the GMP-area to produce urgently required OTC products, like hand sanitizer. Figure 3 of the report also shows that the total number of warning letters decreased for the first time in the last five years. This is a result of a sudden decrease in inspections due to pandemic restrictions, such as those related to travel (for an evaluation of the current inspection backlog see [3]).
How did FDA react to the pandemic?
Furthermore, the FDA describes the impact of the pandemic on-site quality monitoring and pre-approval inspections. FDA made use of several tools to keep this important task running:
- Exploiting Mutual Recognition Agreements with EU and UK to rely on domestic inspections
- Applying the FDA Safety and Innovation Act, which allows the agency to request records or other information from firms in advance of, or in lieu of, an inspection
- Taking into account the FDA surveillance history of firms in order to focus on mission-critical inspections
What will come next?
Finally, the New Inspection Protocol Program (NIPP) should lead to real-world data giving the agency new insights into non-obvious connections between variables and compliance issues. Furthermore, the program will focus directly on Quality Culture, a trend we also observed in recent inspections.
We are eager to see further development in the next months which could shed light on what the mid-term and long-term effects of the pandemic will be. How can they handle the inspection backlog (which is still growing)? And what will the new initiatives bring into the world of pharmaceutical quality and compliance? What is clear is that the effects of the pandemic will continue to shape compliance challenges in the near future.
If you have any questions or discussion points on the report or related topics, please feel free to reach out to me and my colleagues anytime.
[1] FDA; CENTER FOR DRUG EVALUATION AND RESEARCH OFFICE OF PHARMACEUTICAL QUALITY
2020 ANNUAL REPORT – Assuring quality medicines are available to the American public; 2021
https://www.fda.gov/media/145830/download
[2] B. Unger; Part 2: Analysis of FDA FY2019 Drug GMP Warning Letters; 2020
https://redica.com/pharma-part-2-analysis-of-fda-fy2019-drug-gmp-warning-letters/