Personalized mRNA Cancer Immunotherapies: New Guidelines, Big Possibilities
Can you guess the buzzword of the year 2020? It’s mRNA!
Earning Science magazine “Breakthrough of the Year”[1], mRNA vaccines not only reshaped global health but also ignited widespread interest in their potential for personalized medicine. By instructing cells to produce proteins that help the immune system to recognize and fight enemy of interest, mRNA technology is now at the forefront of cancer treatment research. Strikingly, this advancement has been boosted by the parallel evolution of artificial intelligence (AI) and machine learning (ML), which play crucial roles in developing personalized mRNA cancer immunotherapies, also known as “cancer vaccines”. Despite these rapid strides, regulatory frameworks have struggled to keep pace. Since the rise of AI/ML-designed personalized mRNA cancer immunotherapies entering clinical trials, fueled by the rapid development of mRNA technology, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has taken the lead, releasing one of the first draft guidelines to streamline their regulation in early 2025. With neither the FDA nor EMA having issued specific guidance on mRNA-based cancer immunotherapies, the MHRA’s initiative stands as a momentous step in shaping the future of this transformative technology.
Before we dive into MHRA’s draft guideline and their implications for the future of humanity, let’s clarify the concept of “vaccine” and explore how regulatory bodies approach this developing field.
“Vaccines”: Definitions, Classifications, and Beyond
Traditionally, vaccines are preventive measures given to healthy individuals to train the immune system against specific pathogens. Think of your annual flu shot. However, the term “vaccine” has expanded in scientific literature to include therapeutic applications, particularly in cancer treatment. These “cancer vaccines” covers both preventive (like those for HPV and Hepatitis B) and therapeutic vaccines designed to stimulate immune responses in cancer patients.
In the regulatory context, governing agencies acknowledge that while the term “cancer vaccine” is widely used in scientific literature, it is not considered a valid regulatory term. The MHRA defines a vaccine under the Human Medicines Regulations as an antigenic substance used to prevent specific diseases[2], typically involving microorganisms or their derivatives. Similarly, the EU defines vaccines as preparations inducing immunity against infectious agents[3].
As a result, mRNA-based cancer treatments are not considered vaccines even though they teach your body to fight cancer. Instead, they are currently classified as Advanced Therapy Medicinal Products (ATMPs), specifically in a subcategory for gene therapies. For my SME readers, this might sound off, since these mRNA “cancer vaccines” do not actually change your genes like traditional gene therapies do. It’s a bit like calling a floppy disk a Cloud just because they both store data – not quite the right fit, but it’s the box they are put in for now. We’ll explore why this matters later on.
Besides, regulatory bodies prefer to restrict the term “vaccine” to products that boost immunity against infectious diseases. They call cancer treatments “immunotherapies”[4] instead. Such nuanced approach to naming and classification reflects the innovative nature of these therapies and the regulatory challenges they present. It’s a fascinating example of how scientific advancements can push the boundaries of existing regulatory frameworks, necessitating new approaches to ensure both innovation and safety in medical treatments.
Now that we’ve done the groundwork, let’s dive into the MHRA’s latest guideline.
MHRA Draft Guideline on Individualized mRNA Cancer Immunotherapies
This draft guide (public consultation closed on March 31, 2025)[5] outlines the regulation of individualized mRNA cancer immunotherapies which are tailored to each patient’s unique tumor profile using AI/ML. While it’s mainly about cancer treatment, the MHRA envisions that the principles could also work for personalized therapies for other diseases in the future.
Regulatory Scope
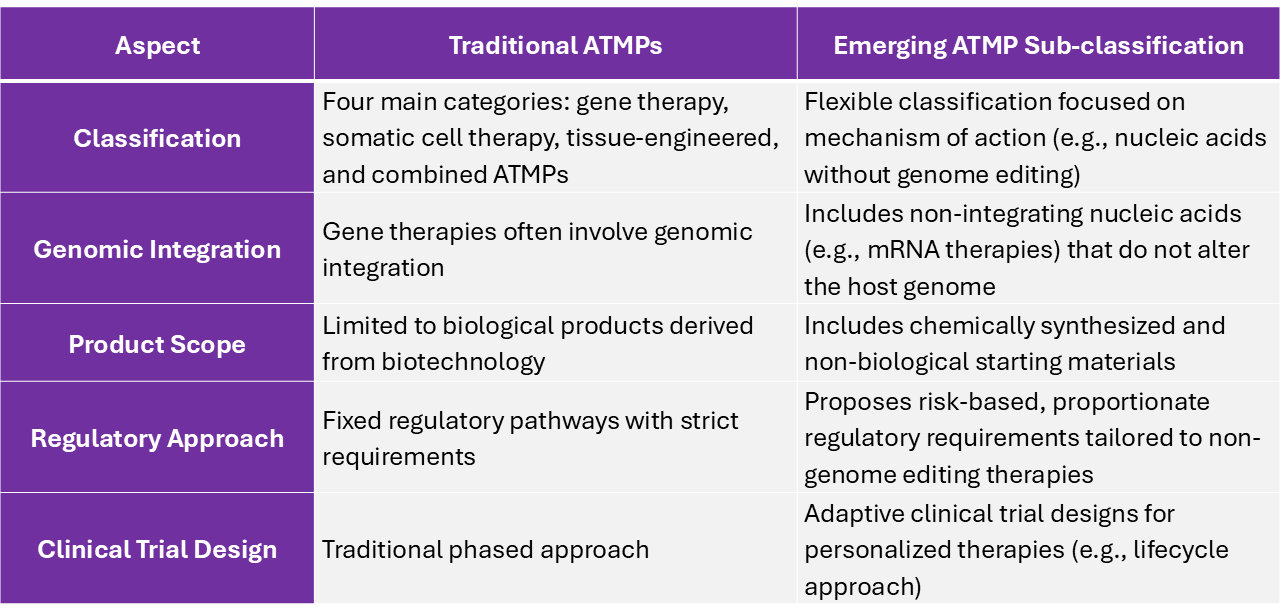
As medical science progresses, regulations must evolve to keep up. The MHRA has proposed new ATMP sub-classification focused on “nucleic acids that do not edit the host’s genome” including mRNA therapies. The table below compares traditional ATMPs with this emerging sub-classification, highlighting key differences in classification, genomic integration, product scope, regulatory approach, and clinical design. It shows how the MHRA is adapting to keep pace with innovative treatments that don’t quite fit the old mold – it’s a bit like updating your iPhone to the latest iOS to send your colleagues live Memojis, rather than basic yellow emojis. Let’s take a look at how these classifications differ:
Table 1. Comparative overview of traditional ATMPs and Emerging ATMP sub-classification (MHRA draft guideline)
Quality Control in mRNA manufacturing
The MHRA draft guideline addresses the unique challenges of manufacturing personalized mRNA cancer therapies, which require tailored batches for individual patients. Unlike traditional mass-produced drugs, these therapies are highly individualized, designed to match a patient’s unique tumor profile. Think of your custom dental crown: just as a dentist creates a precise mold of your tooth to ensure the crown fits perfectly, mRNA manufacturing involves creating a genetic blueprint of tumor cells to produce a treatment specific to the patient. Both processes rely on rigorous quality control to ensure the highest standards. To achieve this, the guideline emphasizes:
- Improved Traceability and Segregation: Maintaining a clear link between patient samples, genetic sequencing, and mRNA selection throughout the process.
- Expanded GMP Application: Applying Good Manufacturing Practice (GMP) to both starting materials and manufacturing processes.
- Comprehensive In-Process Control: Establishing multiple checkpoints throughout the manufacturing pipelines (e.g., tissue sample inspections for contamination, nucleic acid concentration verification before sequencing)
AI/ML in Therapy Design
Artificial intelligence has not only made our everyday lives easier, maybe even a little too easy, but it’s also transformed how we treat cancer. These technologies analyze complex genetic data to find unique markers on cancer cells, called neoantigens, enabling the creation of tailored treatments like individualized mRNA cancer immunotherapies. Not to mention, AI/ML speeds up the process of identifying potential neoantigen “like of the which the world has never seen before”, makes it easier to create personalized treatment faster. However, the application of AI/ML in therapy design brings its own set of regulatory challenges. To tackle these, the MHRA sets out its strategies:
- Regulatory Compliance: May be classified as Software as a Medical Device (SaMD) or regulated under GMP, based on their intended use
- Version Documentation: Developers must record the version of software used for each batch to assess its impact on safety and efficacy
- Data Privacy and Consent: Compliance with data privacy laws and patient consent requirements is mandatory due to the use of patient-derived data
EMA’s Evolving Stance on mRNA Therapy
Alright, Brits. Good work on the guidelines. But what’s brewing in our own backyard?
The EMA has made strides in regulating mRNA therapies, but they are still catching up with personalized mRNA cancer immunotherapies. In June 2023, the EMA released a concept paper on mRNA vaccines for human use, focusing on infectious diseases and leaving out mRNA-based therapeutics for non-infectious diseases like cancer[6].
Despite their initial focus on infectious diseases, the EMA has shown openness to innovative cancer treatments. For example, the agency granted Priority Medicines (PRIME)[7] scheme designation to mRNA-4157/V940, an investigational personalized mRNA cancer immunotherapy developed by Moderna and Merck & Co. for melanoma treatment[8]. This designation underscores how personalized approaches can significantly improve outcomes for specific cancer types.
Fast forward to 2025, the EMA wrapped up a five-year journey –winding up in late January – to finalize its scientific guideline on investigational ATMPs. These guidelines, effective from July 2025[9], represent a shift towards a more flexible regulatory framework. They move away from rigid processes and embrace a risk-based approach that aligns with evolving innovation.
While these changes aren’t specifically aimed at mRNA cancer therapies, they significantly impact the field. We anticipate these regulatory updates will enable innovative approaches such as:
- AI-Driven Neoantigen Validation: Regulators may accept AI-predicted tumor markers and gather real-world evidence over time.
- Personalized Batch Consistency: Regulators could allow some flexibility in the ingredients and production of mRNA therapies (e.g., lipid nanoparticle formulations, patient-specific mRNA sequencing, small-batch manufacturing).
In mid-February 2025, shortly after the MHRA released its draft guideline on individualized mRNA cancer immunotherapies, the EMA’s Committee for Advanced Therapies (CAT) added this draft to its meeting agenda[10]. This move signals potential alignment between EU and UK regulatory approaches for personalized mRNA-based therapies. Additionally, the committee plans to revise its existing Q&A document on gene therapy by Q4 2025. These revisions may include discussions about creating sub-classifications for mRNA-based therapies to better reflect their unique characteristics and mechanisms of action – a proposal also suggested by the MHRA.
Whether intentional or not, on March 31, 2025 – the same day the MHRA closed its consultation period – the EMA launched its public consultation for a draft guideline on the quality aspects of mRNA vaccines[11]. Building on its 2023 concept paper, this new guideline expands and refines quality requirements for mRNA vaccines targeting infectious diseases. While it excludes therapeutic applications like cancer immunotherapies, some of its principles – such as manufacturing processes and analytical controls – may still be relevant for these therapies. The consultation period for this guideline remains open until September 30, 2025.
Looking ahead
As interest in personalized medicine continues to grow, regulatory frameworks are adapting to the unique challenges of mRNA cancer immunotherapies. With the MHRA’s draft guidelines’ consultation period ends, clearer pathways for these treatments are expected. Emerging AI/ML regulations could transform mRNA manufacturing and quality control, impacting everything from neoantigen identification to batch consistency. The EMA’s ongoing evaluation signals a shift towards a more harmonized and risk-based approach, streamlining development and approval across the UK and Europe.
Ready to navigate these changes? GxP-CC is here to guide you through the latest regulatory standards. Contact us today.
References
[2] United Kingdom, Human Medicines Regulations 2012, SI 2012/1916.
[3] Vaccines for human use. European Pharmacopoeia 2012, 7th edition.
[4] Camarero J, Ruiz S. Cancer immunotherapy products: regulatory aspects in the European Union. Hum Vaccin Immunother. 2012 Sep;8(9):1354-9. doi: 10.4161/hv.21142. Epub 2012 Aug 6. PMID: 22863755; PMCID: PMC3579920.
[6] Concept Paper on the Development of a Guideline on the Quality Aspects of mRNA Vaccines. European Medicines Agency. EMA/CHMP/BWP/211968/2023. Published June 22, 2023.
[7] https://www.ema.europa.eu/en/human-regulatory-overview/research-development/prime-priority-medicines
[10] https://www.ema.europa.eu/en/committees/committee-advanced-therapies-cat