Meeting Compliance Goals With Deviation Management And CAPA Systems
Your firm will experience compliance failures from time to time. In the realm of complex GxP-regulated processes, like automated pharmaceutical manufacturing and medical device quality assurance, things can go wrong with little advance warning.
While it would be nice to prevent such issues entirely, it’s far more realistic to implement adaptable deviation management standards. Your ability to do so effectively is crucial to the success of your quality management system as a whole.
What Is Deviation Management?
Deviation management essentially boils down to how well your organization handles aberrations and anomalies. When your batch quality control system fails to catch a run of defective products, for instance, your response in documenting the event and identifying its causes is part of your deviation management strategy.
It’s important to use this stage to classify errors as critical or within acceptable standards and determine whether you’ll need to initiate any FDA Corrective and Preventive Actions as part of your handling process. Having a well defined deviation management strategy may make it easier to identify the tools you’ll need to prevent similar problems in the future.
How Do Deviation Management Implementations Interact?
Using deviation management systems in conjunction with CAPAs and your existing quality management arrangements could be an intelligent way to improve your regulatory compliance. Disjointed management systems can be harder to oversee and maintain, especially for firms that need to stay compliant with varied international regulations or guidelines.
CAPA systems are often cited as highly effective deviation management aids, but they may not be sufficient for all your needs. It’s critical that you evaluate your processes on a per-case basis in order to determine whether a standard CAPA system will be effective. The implementation of an effective CAPA system goes hand in hand with the joint implementation of deviation handling and quality risk management.
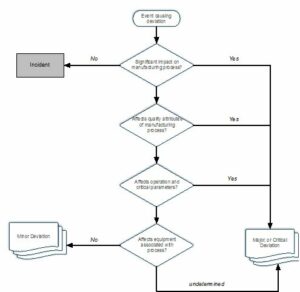
The use of a decision tree allows your employees to have an effective means, by which deviations may be categorized. In such a manner deviations may be categorized into the following types:
- Incidents
- Minor deviations
- Major deviations
- Critical deviations
This is shown in the diagram below.
Although such an implementation will doubtlessly play a vital role in helping your firm maintain FDA compliance, it may not generate the business data you require to meet your internal benchmarks or process-efficiency improvement goals. Working with a consultant could help you build a more expansive system that still meets FDA requirements. Read more about the CAPA process here.
What Can Effective Deviation Management Teach Us?
Like other quality systems, deviation management implementations generally include detailed metrics. These aren’t solely designed to test the efficacy of your processes; they can also be used to evaluate your error handling strategies themselves.
Remember that CAPAs and other error-correcting systems can’t simply be implemented and left to their own devices. They must be validated if they’re to comply with regulations. This is only possible if you create a comprehensive deviation management system that includes thorough failure investigations and sufficient data analysis procedures.
Such management features help you identify whether your error was based on a human mistake or something like an improperly validated IT system. By clarifying the root cause of the problem, you’ll get your firm on track to fix the issue.
Deviation management can be complex. GxP-CC consultants possess the industry and regulatory experience to organize your compliance strategies so that you can implement effective management systems and intelligent oversight. Learn more by contacting them today.