How Compliance-Minded Is Your Organization?
“…was really concerned with quality control failures that was happening really frequently…I would put up error reporting sheets by the machines and it would be taken down. People didn’t want to actually know the number of times that we were having issues…”
“People knew, ‘Don’t speak up or you’ll get fired”
“That was when I was like, this is not an environment, this is not a culture, where they really care about what consequences this might have on patients”
These were just a few quotes from Erika Cheung, a whistleblower from Theranos, the Silicon Valley darling that promised revolutionary finger-prick testing but delivering nothing more than a pinprick of truth [1].
Theranos’ downfall serves as a stark reminder of the critical importance of strong compliance culture in pharma and biotech industries. This cautionary tale underscores a valuable lesson: innovation in healthcare thrives on transparency and data integrity. As for industry leaders, the message is clear –a robust compliance-minded culture isn’t about sidestepping legal pitfall. It’s about safeguarding patients and delivering on the promise of life-changing medical advancement.
So, how can your organization foster a culture that prioritizes compliance and integrity at every turn?
What is A Compliance-Minded Culture? The Overlooked Pillar of Data Integrity
A compliance-minded culture is a foundation of strong quality culture and data integrity in pharma and biotech industries. It refers to the shared value, beliefs, attitudes, and behaviors that promotes regulatory adherence, preventive risk management, and ethical practices within an organization. The culture drives that regulatory requirements are not just met but proactively integrated into every aspect of operations.
While many pharmaceutical companies prioritize the technical aspect of data integrity—processes, systems, and procedures—human factor is often underestimated. A robust quality culture [2] optimizes pharmaceutical operations by emphasizing prevention, patient safety, and continuous improvement. Within this comprehensive framework, a compliance-minded culture serves as a key element as even most sophisticated systems can fail without the right people, mindset, and culture in place.
Yet, several challenges continue to emerge and derail the progress:
- An increasing complex and rapidly evolving regulatory landscape
- Balancing innovation with compliance demands
- Corporate cultural barriers, including fear of retaliation
- High turnover rates and the need for specialized compliance talents
By navigating these challenges and embrace a culture that values compliance at all levels, from floor to the C-suite, organizations can build a resilient foundation to support technical pillar of data integrity.
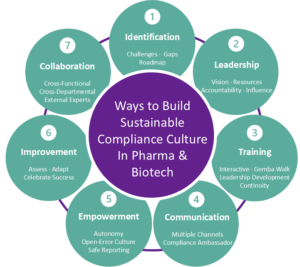
How to Build (and Improve) your Compliance-Minded Culture
- Identify current challenges and key risk areas through a compliance check-up and prioritize compliance efforts accordingly; develop a targeted compliance roadmap that addresses specific gaps and aligns with organizational goals.
- Cultivate strong compliance leadership by establishing a clear compliance vision; led by example; provide necessary resources; and ensure accountability throughout the organization.
- Enhance compliance training programs with interactive approaches (e.g., gamification); regular Gemba Walks to connect trainers with frontline staff; continuous learning initiatives (e.g., dedicated learning hours); and leadership development focused on addressing retaliation and providing guidance, fostering a comprehensive and engaging learning environment.
- Maintain continuous communication by utilizing multiple channels to share regulatory updates and launch a Compliance Ambassador Program to facilitate three-way communication between quality, IT, and operational team.
- Empower employees by granting employees with appropriate decision-making authority; offering safe channels for voicing concerns; implementing recognition programs for compliance excellence; and promoting an open error culture that encourages learning from mistakes.
- Drive continuous improvement through regular assessments using surveys and tools; adapting strategies based on feedback and evolving regulations; and celebrating compliance successes to boost ongoing enhancement.
- Actively engage in collaboration by leveraging external experts like GxP-CC and promoting cross-functional and departmental teamwork internally to enhance compliance strategies.
The Path Forward
Succeeding in pharma and biotech industries involves more than just meeting regulatory requirements. While we can’t say for certain that a strong compliance culture would have prevented Theranos’ downfall, implementing key strategies could have mitigated compliance risks. It’s tempting to believe that extreme cases like Theranos are unlikely to occur close to home, but the reality is that cultural issues can arise anywhere, often in more subtle forms.
To avoid becoming the next cautionary tale, organizations should focus on identify current challenges, cultivating strong compliance leadership, maintaining continuous communication within organization and departments, enhancing compliance training programs, empowering employees by promoting an open error culture, driving continuous improvements, and actively engage in collaboration. By incorporating these elements into daily practices, one can establish a strong and ethical business culture that is both resilient and compliant.
Remember, a robust compliance-minded culture isn’t about sidestepping legal pitfall. It’s about building a solid foundation for your organization to stand on.
Want to learn more? Or do you feel overwhelmed and unsure of where to start? Contact GxP-CC today and let us help you to strengthen your compliance culture and safeguard both your patients and your business. Together we can ensure that innovation in healthcare thrives on a foundation of transparency and data integrity.